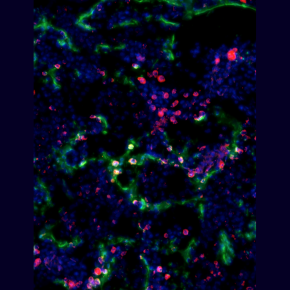
Discovery of a transient wave of perinatal hematopoiesis through endothelial-hematopoietic transition in vertebrates.
One of the main challenges of regenerative medicine is to produce hematopoietic stem cells (HSCs). In collaboration with the Ubrecht Institute (Netherlands), researchers are demonstrating the existence of a new hematopoietic wave from resident hemogenic endothelial cells in the bone marrow of the late fetus and young adult. This wave joins the end of embryonic hematopoiesis with the beginning of medullar hematopoiesis in chicken and mice. The results of this study are published in the journal Nature Cell Biology.
HSC transplants are the therapeutic response to the treatment of certain malignant hematological diseases as well as to the radio or chemotherapy care required to treat solid tumors. The controlled production of HSC from pluripotent precursors remains very difficult to achieve in vitro and therefore requires a better understanding of HSC production during development.
Hematopoietic stem cells (HSCs) are multipotent cells that reside in the bone marrow (BM) of adults and are responsible for the daily production of all hematopoietic cells.
The embryo develops using a set of hematopoietic cells from an embryonic appendage, the yolk sac, and this production is necessary and sufficient to ensure its survival. The first HSCs are produced very early during development, in small numbers from specialized endothelial cells, called hemogenic (HECs), via a cellular transition, designated as endothelio-hematopoietic (EHT), which occurs in the embryo aorta. This small pool of HSCs is then amplified in the fetal liver before colonizing, at the end of gestation, the bone marrow (BM) via a process that will see its full functioning a few weeks after birth.
To determine how the fetus and young adult fill this "hematopoietic depression", the researchers used a combination of experimental, genetic, transcriptomic and functional embryology approaches using the chicken and mouse models. Using microsurgery, genetic markings and in vivo imaging, they identified somites as sources of the BM vascular network. Unexpectedly, they found that some endothelial cells derived from somites had blood-generating potential, i.e. cells capable of producing HSCs and multipotent hematopoietic progenitors in the late fetus / young adult via EHT. The hematopoietic cells resulting from this EHT are characterized by a specific molecular signature close to endothelial cells undergoing EHT and/or HSCs recently formed in the embryonic aorta. They show prominent expression of some members of the Notch signaling pathway, specific endothelial genes and transcription factors involved in EHT.
These results therefore demonstrate the existence of a perinatal transient hematopoietic production. HSCs and hematopoietic progenitors can be generated de novo during late embryonic stages or after birth, from blood endothelial cells of somitic origin via a EHT similar to that produced in the embryo aorta. In addition, this transient hematopoietic wave could also help prepare BM niches to host HSCs amplified in the fetal liver.
The identification of all stages of hematopoietic stem cell genesis and the molecular events controlling it is of fundamental interest and could open up avenues for the therapy of hematopoietic disorders.
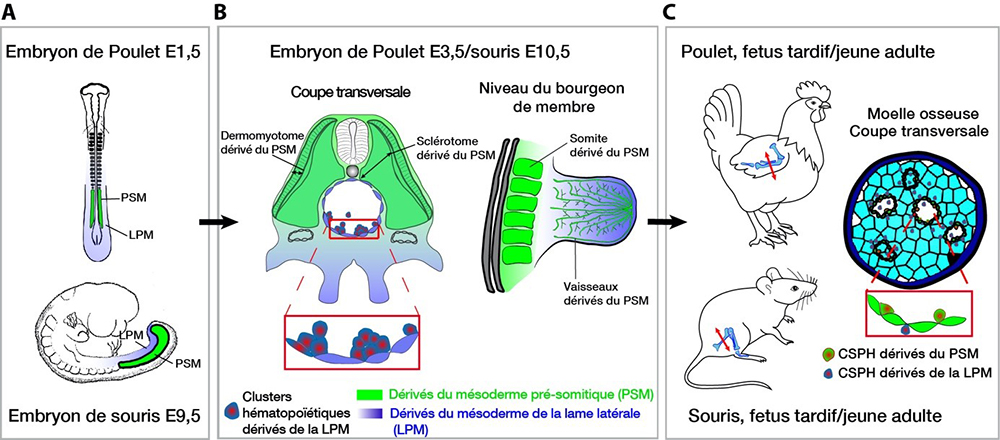
Figure : A-C: Location and fate of the lateral plate mesoderm (LPM, blue) and pre-somitic mesoderm (PSM, green) at three times representative and equivalent of chicken and mouse development. A: PSM and LPM in mouse embryos embryonic days (E)1.5 and E9. LPM: mesoderm located laterally and ventrally with respect to the PSM. B: The PSM is subdivided into somites, which give rise to muscles, vessels and vertebrae. The hemogenic ECs of the aorta and associated hematopoietic clusters are exclusively from the LPM. C: In young adults, bone and marrow come from the LPM, the vascular system of the PSM. Most hematopoietic stem cells and hematopoietic progenitors (HSPCs) in the bone marrow come from the aorta. We show a de novo production by the endothelium of the bone marrow derived from PSM.
In vivo generation of haematopoietic stem/progenitor cells from bone marrow-derived haemogenic endothelium
Yvernogeau L, Gautier R, Petit L, Khoury H, Relaix F, Ribes V, Sang H, Charbord P, Michèle Souyri M, Robin C, Jaffredo T
Nature Cell Biology, 4 Nov 2019 https://doi.org/10.1038/s41556-019-0410-6